ibidi Pump System
A pump system for long-term cell culture under flow to mimic physiologic conditions
System includes:
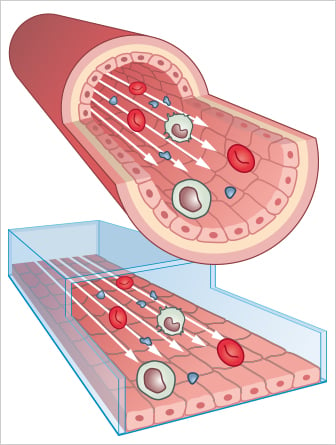
Many cell types experience moving fluids, such as vascular and lymphatic endothelial cells or kidney and lung epithelial cells. This liquid flow causes shear stress, a mechanical force that significantly impacts the cells’ physiological behavior, structural properties, and function. Cultivating cells under flow in vitro enables studying endothelial and epithelial cells in a more physiological, in vivo-like environment.


With the ibidi Pump System Quad with four Fluidic Units, ibidi also offers a high-throughput solution for keeping multiple µ-Slides under flow.

Please find more detailed information about the planning, conduction, and data analysis of cell culture under flow assays here.
Download the “Cell Culture Under Flow” Application Guide as a PDF here.
* Depending on the Perfusion Set and channel slide used, find an overview here.
Check out our online courses to learn more:


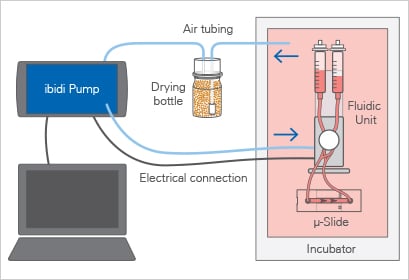
The ibidi Pump System consists of two main components: the ibidi Pump and the Fluidic Unit.
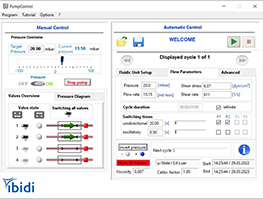
The ibidi Pump is an air pressure generator that is computer-controlled and designed to sit outside the incubator. While inside the incubator, the Fluidic Unit performs the valve-switching operations on the Perfusion Set (fluidic reservoirs and tubing) to generate unidirectional flow in a channel slide. The standardized Luer connectors make the system compatible with any flow or channel slide. The ibidi Pump connects to the Fluidic Unit with an electrical cable and air pressure tubing. The PumpControl Software gives the ability to automatically program complex flow patterns, including calculating the shear stress acting on the cells.
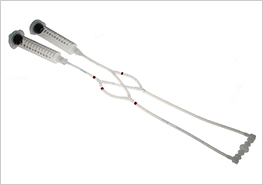
The use of pinch valves allows the closed circuit of the cell culture medium to be set up in the laminar flow hood under sterile conditions since only sterilized, disposable parts are in contact with the medium. The setup can be easily transferred to the microscope, the incubator, or the stage top incubator without compromising its sterility.
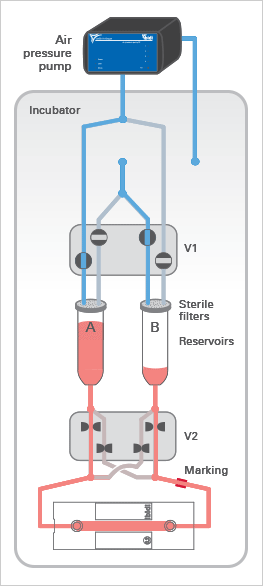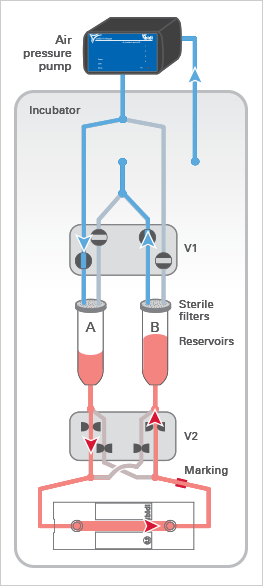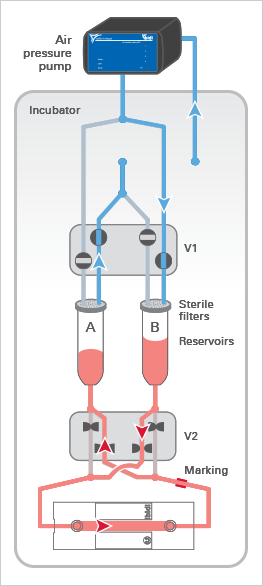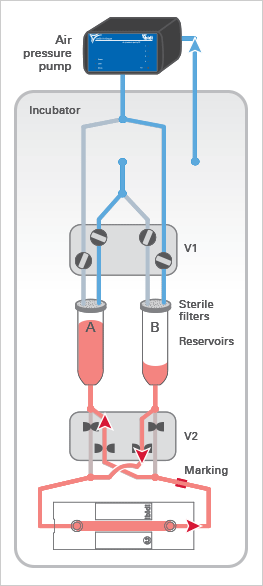
The ibidi Pump applies pressurized air (in blue) to the reservoirs of the Fluidic Unit, which moves the medium and generates the flow in the channel of the µ-Slide. The medium (in red) only moves in the reservoirs and tubing of the mounted Perfusion Set and does not get in contact with the valves, thereby maintaining sterility.
The Fluidic Unit’s active components are the two switching valves V1 and V2. Valve V1 directs the air pressure to either reservoir A or B, pumping medium back and forth between the reservoirs.
Valve V2 is switched simultaneously to ensure a unidirectional laminar flow in the channel. Due to the Perfusion Set’s crisscrossed tubing within valve V2, the medium is always directed to the same end of the channel slide (see arrows).

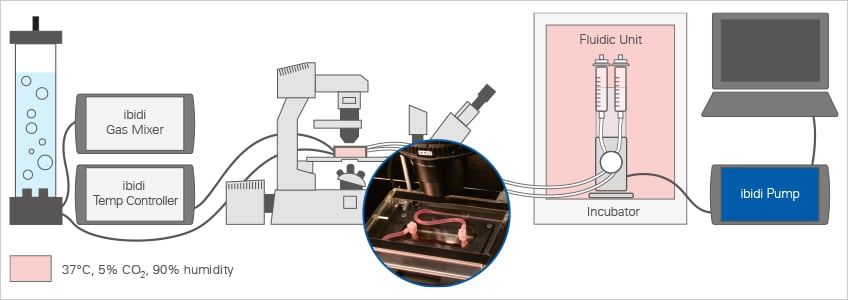
For live cell microscopy experiments under flow, the ibidi Pump System can be easily combined with the ibidi Stage Top Incubator Slide/Dish – Silver Line. This setup allows for long-term perfusion assays directly on the microscope with defined shear stress, temperature, humidity, and CO2 (and, optionally, O2) levels.
ibidi offers a variety of channel slides for culturing cells under flow. The following ibidi µ-Slides can be attached to the ibidi Pump System:
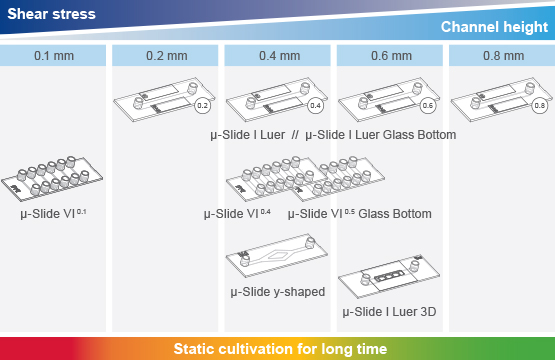
Different experimental setups require different µ-Slide geometries and shear stress ranges. The possible shear stress range achieved with the ibidi Pump System depends on the combination of the Perfusion Set and µ-Slide. Details can be found in our Perfusion Set and µ-Slide Selection Guide.

Perfusion Set and µ-Slide Selection Guide
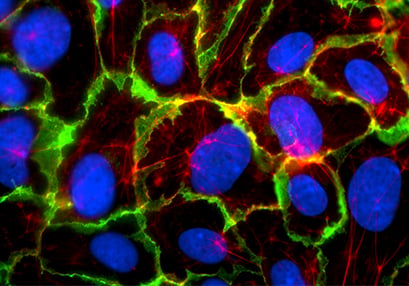
After the flow experiment, immunofluorescence stainings can easily be performed in the ibidi Channel Slides. When comparing the cultivation of HUVEC under static and flow conditions, the differences in the cellular organization and various cellular compartments (e.g., tight junctions) are clearly visible.
Static culture
Flow culture
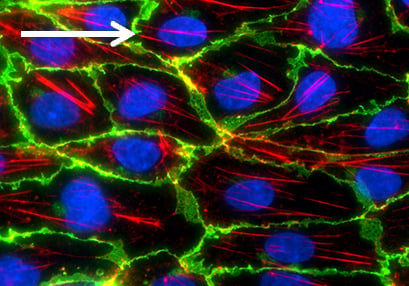
Comparison of HUVEC (human umbilical vein endothelial cells) structure under static and flow conditions using fluorescence microscopy. Left: cell structure after 5 days of static culture (0 dyn/cm²) in the µ-Dish 35 mm, high ibiTreat. Right: cell structure after 5 days of flow culture (10 dyn/cm² using the ibidi Pump System) in the µ-Slide I 0.4 Luer, ibiTreat. Under static conditions, HUVEC are generally large with a chaotically structured actin skeleton. In contrast, flow-conditioned cells are elongated and show distinct F-actin stress fibers (stained with phalloidin, red). VE-cadherins (green), which mark the adherence junctions, are present in both conditions. Nuclei are stained with DAPI (blue).
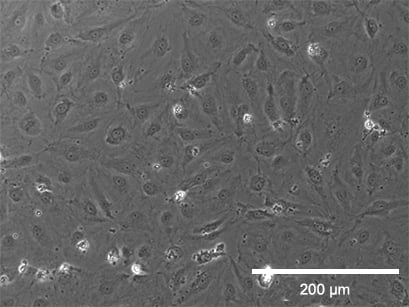
Static culture

Flow culture
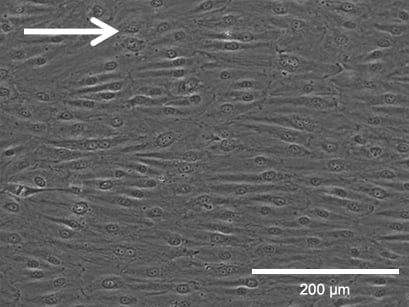
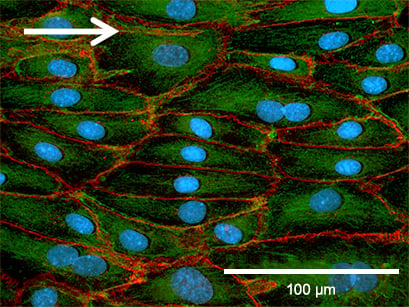
Phase contrast (top) and fluorescence microscopy (bottom) of Human Pulmonary Microvascular Endothelial Cells (HPMECs). Static culture (left) and flow culture at 2.3 dym/cm² using the ibidi Pump System (right) after 72 h. Green: β-Actin, red = VE-Cadherin, blue = DAPI. Data by Daniel Bourquain, Robert Koch Institut, Berlin, Germany.

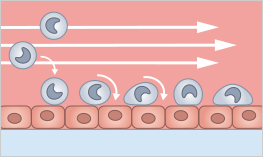
Rolling and adhesion of polymorphonuclear leukocytes on LPS-stimulated cerebrovascular endothelial cells (CVEC) in the µ-Slide VI 0.4. Granulocytes were perfused at 1 dym/cm² using the ibidi Pump System. Data by Gediminas Cepinskas, Ontario, Canada.



115 ร่มโพธิ์แมนชั่น ห้องเอ 1 ถนนริมทางรถไฟสายปากน้ำ
แขวงคลองเตย เขตคลองเตย กรุงเทพมหานคร 10110