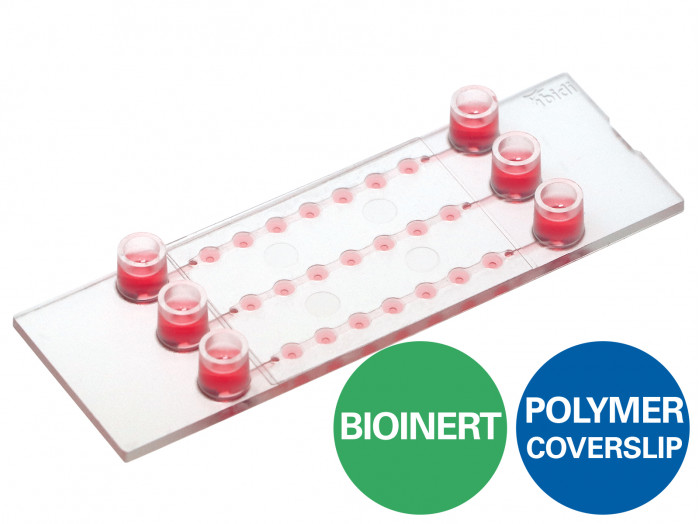
ibidi : µ-Slide Spheroid Perfusion
ช่อง µ-Slide ที่ฉีดได้พร้อม 3 x 7 หลุมสำหรับการเพาะปลูกลูกทรงกลมในระยะยาวและกล้องจุลทรรศน์ระดับไฮเอนด์
- เหมาะอย่างยิ่งสำหรับการเพาะปลูกในระยะยาวและการแพร่กระจายของ 3D spheroids หรือ organoids บนพื้นผิว Bioinert
- การเตรียมการที่ยืดหยุ่นสำหรับการแพร่กระจาย: ทรงกลมสามารถถ่ายโอนหรือสร้างได้โดยตรงในบ่อน้ำ
- ห้องภาพที่มีคุณภาพออปติคอลที่ยอดเยี่ยมสำหรับกล้องจุลทรรศน์ความละเอียดสูง
การใช้งาน
- การเพาะปลูกและกล้องจุลทรรศน์ความละเอียดสูงของการรวมตัวของเซลล์สามมิติ (เช่น ทรงกลมและออร์แกนอยด์)
- สำหรับใช้กับระบบปั๊ม ibidi หรืออุปกรณ์ปั๊มอื่น ๆ ที่มีขั้วต่อ Luer
- การแพร่กระจายของทรงกลมสำหรับการจัดหาอาหารสดในระหว่างการเพาะปลูกระยะยาวและการเข้าถึงจลนพลศาสตร์การเจริญเติบโตของทรงกลม
- การสร้างทรงกลมโดยตรงในบ่อน้ำ
- การดึงข้อมูลทรงกลมสำหรับการประมวลผลขั้นปลาย (เช่น อิมมูโนฟลูออเรสเซนส์ จุลกายวิภาค และการวิเคราะห์ทางชีวเคมี)
- การแพร่กระจายของเซลล์โมโนเลเยอร์ (เซลล์เกาะติด), เซลล์แขวนลอย หรือวัฒนธรรมร่วม
- การตั้งค่า Organ-on-a-chip พร้อมเมแทบอลิซึมต้นน้ำและปลายน้ำ
- การถ่ายภาพเซลล์แบบสดและกล้องจุลทรรศน์ของการรวมตัวของเซลล์ 3 มิติ
- การย้อมสีอิมมูโนฟลูออเรสเซนส์และกล้องจุลทรรศน์เรืองแสงที่มีความละเอียดสูงของเซลล์ที่มีชีวิตและเซลล์ตายตัวและการรวมตัวของเซลล์
A perfusable channel µ-Slide with 3 x 7 wells for long-term spheroid cultivation and high-end microscopy
- Ideal for the long-term cultivation and perfusion of 3D spheroids or organoids on the Bioinert surface
- Flexible preparation for perfusion: spheroids can be transferred or created directly in the wells
- Imaging chamber with excellent optical quality for high-resolution microscopy
- Surface Modification: Uncoated: #1.5 polymer coverslip, hydrophobic, sterilized
Pcs./Box: 15 (individually packed) - Surface Modification: ibiTreat: #1.5 polymer coverslip, tissue culture treated, sterilized
Pcs./Box: 15 (individually packed) - Pcs./Box: 15 (individually packed)
Surface Modification: Bioinert: #1.5 polymer coverslip, surface passivation with Bioinert, sterilized
Applications
- Cultivation and high-resolution microscopy of three-dimensional cell aggregates (e.g., spheroids and organoids)
- For use with the ibidi Pump System or any other pump device with Luer connectors
- Perfusion of spheroids for fresh medium supply during long-term cultivation and access to spheroid growth kinetics
- Spheroid generation directly in the wells
- Spheroid retrieval for downstream processing (e.g., immunofluorescence, histology, and biochemical assays)
- Perfusion of cell monolayers (adherent cells), suspension cells, or co-cultures
- Organ-on-a-chip setups with up- and downstream metabolization
- Live cell imaging and microscopy of 3D cell aggregates
- Immunofluorescence staining and high-resolution fluorescence microscopy of living and fixed cells and cell aggregates
Specifications
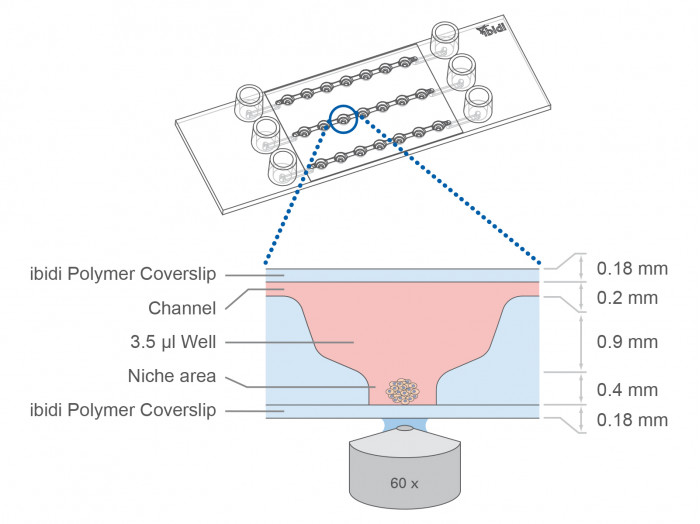
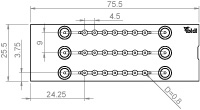
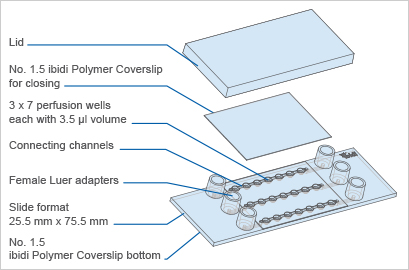
| Outer dimensions (w x l) | 25.5 x 75.5 mm² |
| Number of channels | 3 |
| Number of wells | 3 x 7 |
| Volume per well | 3.5 μl |
| Well height (bottom niche) | 0.4 mm |
| Well height (total) | 1.3 mm |
| Well diameter (bottom) | 0.8 mm |
| Channel volume (total) | 45 µl |
| Channel height | 0.2 mm |
| Channel width | 1.0 mm |
| Growth area per well | 0.5 mm² |
| Coating area using 3.5 µl | 9.7 mm² |
| Adapters | Female Luer |
| Volume per reservoir | 60 µl |
| Top cover: ibidi Polymer Coverslip | |
| Bottom: ibidi Polymer Coverslip | |
Technical Drawing
Technical Features
- Optimized well geometry for spheroid/organoid culture and microscopy
- Analysis of up to 21 samples in one slide
- Perfusion can be applied for optimal nutrient supply during the cultivation of 3D cell aggregates
- Initial open well format allows for easy sample preparation—easily close the wells with a coverslip for the application of flow
- Channels create a simple fluidic connection of the wells
- Luer adapters enable easy pump connection (e.g., to the ibidi Pump System)
- Available with three surfaces:
- Sample can be observed through the ibidi Polymer Coverslip bottom using high-resolution fluorescence microscopy
- Compatible with staining and fixation solutions
- Compatible with differential interference contrast (DIC) microscopy when used with a DIC lid
- Made of fully biocompatible materials

Find Out More
Please find more detailed information about 3D cell culture assays here.
Download the “3D Cell Culture” Application Guide as a PDF here.
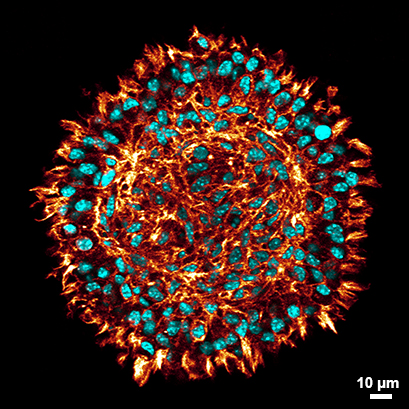
Confocal microscopy of a cross-section of a stained L929 spheroid after clearing (red: phalloidin, cyan: DAPI) in the µ-Slide Spheroid Perfusion. Clearing of the spheroids allows the visualization of cells even at the center of the spheroid fibroblasts while at the same time preserving the spheroid`s morphology. Find detailed information in the User Protocol 11: “Protocol for Spheroid Culture, Staining, and Clearing for 3D Imaging”. Data by Julian Hofmann and Selina J. Keppler, Technical University Munich, Germany.
The Principle of the µ-Slide Spheroid Perfusion
Optimal Nutrition Through Specialized Geometry
The µ-Slide Spheroid Perfusion is a specialized flow chamber for culturing free-floating 3D aggregates. It consists of 3 x 7 flat-bottomed wells, which are connected through a channel above. Each well forms its own niche, where the specimen is cultured.
When applying perfusion through the channel, fresh medium continuously diffuses to the specimen. This ensures optimal nutrition and oxygen diffusion throughout the experiment without that the specimen is exposed to significant shear forces.
The setup guarantees maximum of viability, but with minimum of shear stress for spheroids, organoids, or tissue.


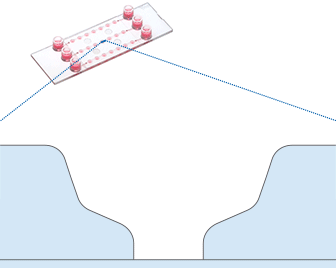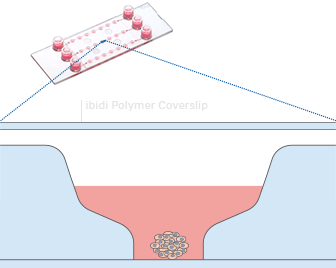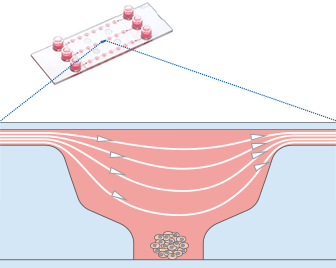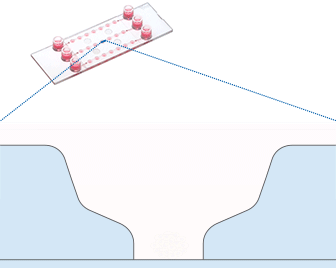
Shown here is a computational fluid dynamics (CFD) simulation in the single wells of the µ-Slide Spheroid Perfusion. The fluidic characteristic of each well is optimized for specimens in the bottom niche. Please note that the shear forces are dominant in the upper well area while the niche is protected.

High-Quality Imaging Through the Coverslip Bottom
The µ-Slide Spheroid Perfusion comes with a thin ibidi Polymer Coverslip Bottom that has the highest optical quality (comparable to glass), and is ideally suitable for high-resolution microscopy. The compatibility with oil immersion microscopy and fluorescence imaging makes the µ-Slide Spheroid Perfusion the ideal spheroid imaging chamber.
Find more information and technical details about the coverslip bottom of the ibidi chambers here.
Experimental Workflow
The µ-Slide Spheroid Perfusion is a revolutionary technology that creates ideal conditions for spheroids, organoids, or tissue. It also allows for their simultaneous cultivation and imaging.
The µ-Slide Spheroid Perfusion was designed for cell cultivation under perfusion. Cultivating cells under perfusion has been shown to:
- Enable optimal nutrition and gas diffusion
- Reduce apoptosis and necrosis
- Increase spheroid size and lifespan
- Enable excellent viability and proliferation in long-term culture
- Improve maturation
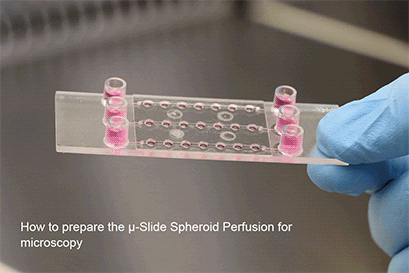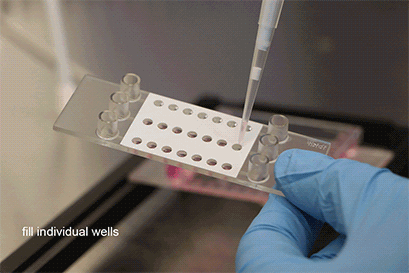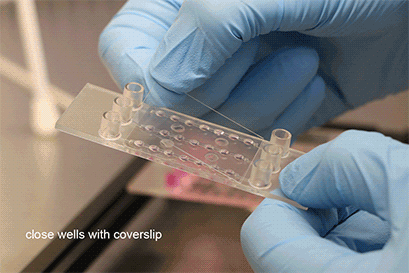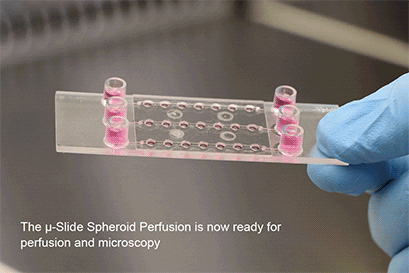
Wei, L., Li, W., Entcheva, E., & Li, Z. (2020). Microfluidics-enabled 96-well perfusion system for high-throughput tissue engineering and long-term all-optical electrophysiology. Lab on a Chip, 20(21), 4031. 10.1039/D0LC00615G
Read article
Nashimoto, Y., Okada, R., Hanada, S., Arima, Y., Nishiyama, K., Miura, T., & Yokokawa, R. (2020). Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials, 10.1016/J.BIOMATERIALS.2019.119547.
Read article
Wan, X., Li, Z., Ye, H., & Cui, Z. (2016). Three-dimensional perfused tumour spheroid model for anti-cancer drug screening. Biotechnology Letters, 38(8), 1389. 10.1007/S10529-016-2035-1.
Read article
Seeding Pre-Formed Spheroids
With a simple workflow, existing 3D cell aggregates are easily placed into the well using a pipette. After closing the wells, the channel is filled with culture medium and the perfusion is started using the ibidi Pump System or any other cell culture pump. The spheroids can be generated using all common protocols and methods (e.g., hanging drop, liquid-overlay, ULA plates, or bioreactors). The µ-Slide Spheroid Perfusion itself does not need any embedding substrates, scaffolds, or supportive structures.
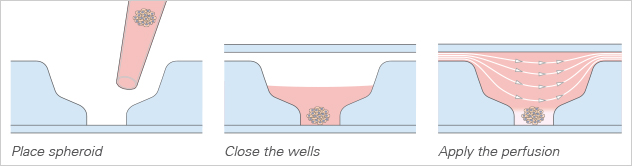
Spheroid Generation Inside the Wells
Alternatively, spheroids can be generated directly inside the wells of the µ-Slide Spheroid Perfusion with the Bioinert surface. This self-organization process is an easy and time-saving set-up for generating homogeneous spheroids in each single well.

The three-dimensional cell aggregates can easily be retrieved for downstream applications, such as histology, protein profiling, and further cultivation.
Which Surface Should I Use for My Assay?
| Bioinert | Uncoated | ibiTreat | |
| Surface type | Passivation | Hydrophobic | Tissue culture-treated, hydrophilic |
| Recommended cell types | Organoids and spheroids, cell aggregates, suspension cells | Suspension cells | Adherent cells |
| Cell adhesion | No | Hardly | Yes |
| Protein adsorption | No | Yes | Yes |
The Bioinert Surface
Bioinert is the most suitable surface modification of the ibidi Polymer Coverslip for culturing spheroids and suspension cells, because it provides complete passivation. With the µ-Slide Spheroid Perfusion Bioinert, the 3D aggregates are free-floating. Unlike ibiTreat and Uncoated, the Bioinert surface cannot bind any proteins, so cell adhesion is impossible—even in long-term experiments.
The Uncoated Surface
Uncoated is a hydrophobic surface variation of the ibidi Polymer Coverslip. Without an additional protein coating, adherent cells hardly attach to this surface. With the µ-Slide Spheroid Perfusion, the Uncoated surface can be used for assays with suspension cells. To avoid any protein adsorption, we recommend using the Bioinert surface.
The ibiTreat Surface
ibiTreat (tissue culture-treated) is the hydrophilic surface modification of the ibidi Polymer Coverslip. A vast majority of adherent cells grow well on it without the need for an additional protein coating. Because of the good cell attachment properties, ibiTreat is not suitable for preventing spheroids/organoids from attaching to the bottom in the µ-Slide Spheroid Perfusion.
Find more information about the different surfaces of the ibidi chambers here.
Applications of the µ-Slide Spheroid Perfusion
The µ-Slide Spheroid Perfusion is an easy-to-use microfluidic bioreactor that is suitable for a variety of applications with multicellular 3D aggregates. Alternatively, single cells can be used as suspension culture or as a cell monolayer when attached to the well bottom. Moreover, multiple cell types can be combined in one well to create a long-term co-cultivation system. The revolutionary technology of the µ-Slide Spheroid Perfusion offers an efficient and reproducible approach that can simultaneously grow and image your specimen.
Please note: the µ-Slide Spheroid Perfusion is available with three different surfaces. Please consult this table to determine the optimal surface for your application.

Cell Types and Applications
The µ-Slide Spheroid Perfusion is compatible with pre-formed 3D aggregates and can be used with any protocol or method (e.g., hanging drop, liquid-overlay, ultra-low attachment (ULA) plates, or bioreactors). This makes the µ-Slide Spheroid Perfusion suitable for a large variety of cell types and applications, such as:
- Multicellular cancer spheroids and tumor cells
- Creation of organoids from pluripotent or adult stem cells
- Differentiation and cultivation of organoids
- Human induced pluripotent stem cells (iPSCs)
- Embryoid bodies (EBs)
- Neuronal stem cells (NSC) aggregates (neurospheres)
- Hepatitis virus research models
Long-Term Culture of Spheroids
The µ-Slide Spheroid Perfusion overcomes any technical limitations encountered when working with long-term spheroid cultures. It offers an efficient, standardizable way to generate and access multicellular spheroids, which makes it a versatile tool.
3D Culture of Cancer Cells
The µ-Slide Spheroid Perfusion offers an efficient and reproducible approach to growing and maintaining cancer cells. The optimal nutrition and gas diffusion capabilities of the assay allow for deeper insights into high metabolism cell types, such as primary tumor cells in a 3D in vitro model.
3D Stem Cell Aggregation
Pluripotent stem cells can be directly inoculated as single cells into the wells of the µ-Slide Spheroid Perfusion. During the self-aggregation phase, the cells will form homogeneous aggregates. At the same time, live cell imaging can be used to gain further details by using high resolution microscopy. The resulting stem cell 3D aggregates can be retrieved directly for further differentiation (e.g., organoids or any other downstream application).
Co-Culture of Different Cell Types
For cell-based drug discovery and toxicity testing, co-cultures can be set up easily in the µ-Slide Spheroid Perfusion. Up- or downstream metabolization studies with co-cultures can be performed within one channel or by connecting several channels to a microfluidic bioreactor.

Application Examples
Highly Improved Spheroid Growth Rates When Cultured Under Perfusion

L929 fibroblasts show spheroid formation in the µ-Slide Spheroid Perfusion, Bioinert, days 1–14, seeding concentration 5 x 105 single cells/ml. Left: no perfusion, medium exchange every second day. Right: perfusion with the ibidi Pump System, 0.75 ml/min. Phase contrast microscopy, 10x objective lens, well diameter 800 µm.
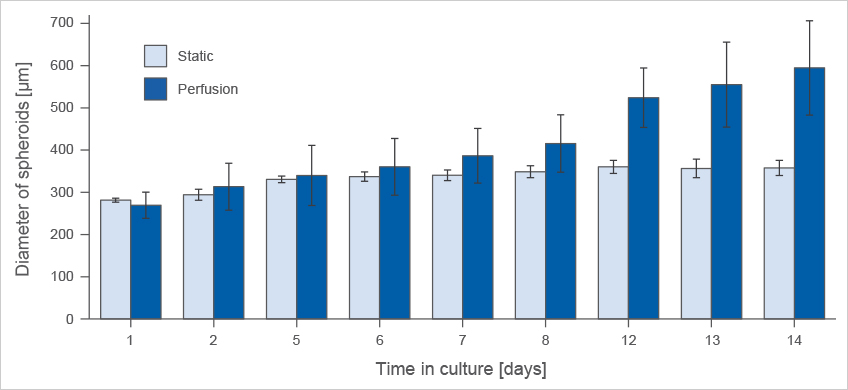
Comparison of spheroid formation of L929 fibroblasts in the µ-Slide Spheroid Perfusion, Bioinert, seeding concentration 5 x 105 cells/ml.
Spheroid Formation of MCF-7 Breast Cancer Cells
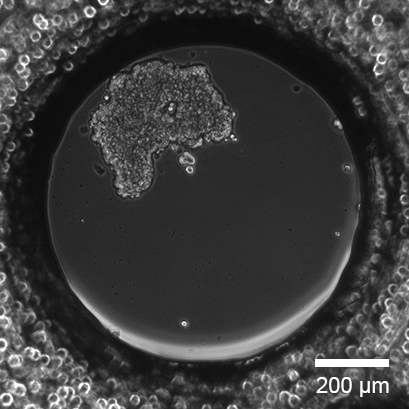
Single cell seeding of MCF-7 breast cancer cells with spheroid formation in the µ-Slide Spheroid Perfusion, Bioinert, 1 day after seeding. Seeding concentration 5 x 105 cells/ml. Phase contrast microscopy, 10x objective lens.
Live/Dead Staining of a Long-Term Cultured Spheroid
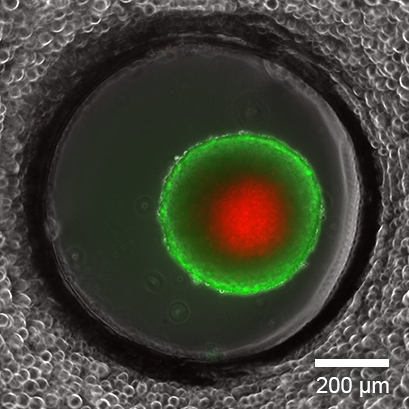
Live/dead FDA/PI staining of an L929 spheroid in the µ-Slide Spheroid Perfusion, Bioinert, after 14 days in culture with perfusion using the ibidi Pump System, 0.75 ml/min. Green: living cells (fluorescein diacetate, FDA); red: dead cells (propidium iodide, PI). Widefield fluorescence microscopy, 10x objective lens.
Fluorescence Microscopy of Adherent Cells
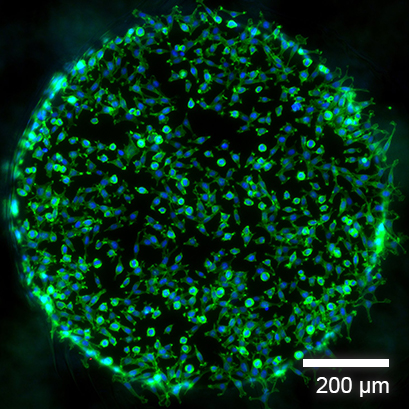
Fluorescence imaging of L929 fibroblasts in the µ-Slide Spheroid Perfusion, ibiTreat, fixated 2 hours after seeding. Green: F-actin (phalloidin); blue: nuclei (DAPI). Widefield fluorescence microscopy, 10x objective lens.
Organoid Co-Culture of PDAC Cells and Fibroblasts
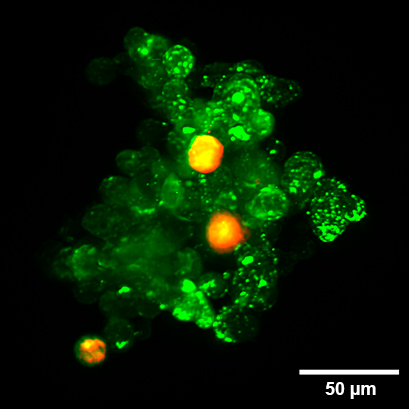
Organoid co-culture of the human pancreatic cancer (PDAC) cell line PA-TU-8988T (green, stained with CellTrackerTM Green) and the murine fibroblast cell line mPSC4 (red, stained with CellTrackerTM Orange CMTMR) in the µ-Slide Spheroid Perfusion. The µ-Slide was covered with a 25 µm FEP foil for matching the refractive index closer to water during upright light sheet microscopy. The image was acquired by S. Volkery at MPI Muenster with the M Squared Aurora Airy beam upright light sheet setup. The sample was provided by K. Roth, University Marburg, Germany.
Which Slide Should I Use for My Application?
| µ-Slide Spheroid Perfusion | µ-Slide III 3D Perfusion | µ-Slide I Luer 3D | µ-Slide I Luer | |
| Application | ||||
| Perfusion of samples | Yes | Yes | Yes | Yes |
| Defined shear stress on cell monolayers | No | No | Yes, on gel matrix | Yes, on coverslip |
| Gel matrices for 3D | No | Yes | Yes | No |
| Cell Type | ||||
| Spheroids/organoids | Yes, free floating in well | Yes, inside gel matrix only | Yes, inside gel matrix only | No |
| Suspension cells | Yes, free floating in well | Yes, inside gel matrix only | Yes, inside gel matrix only | Yes, in flow suspension only |
| Adherent cells | Yes, on coverslip | Yes, inside or on gel matrix | Yes, inside or on gel matrix | Yes, on coverslip |