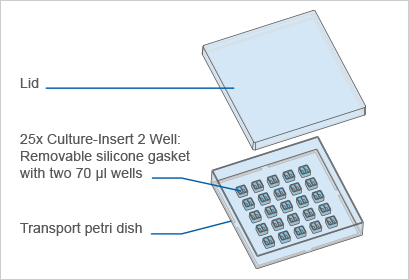
ซิลิโคนแบบ 2 ช่อง เพื่อวางบนจานเลี้ยงเซลล์ – สามารถกำหนดช่องว่างระหว่างเซลล์ สำหรับการทดลองแบบ wound healing, การทำ cell migration, การทำ 2D invasion หรือการเลี้ยงเซลล์
- เหมาะสำหรับการทดลองแบบ wound healing
- สามารถทำการทดลองซ้ำได้เนื่องจากช่องว่างของส่วนที่เป็นซิลิโคนมีขนาด 500 µm เท่ากันทุกชิ้น โดยซิลิโคนติดแน่นกับก้นจานมั่นใจได้ว่าไม่มีการรั่วซึมของอาหารเลี้ยงเซลล์
- มีซิลิโคนจำนวน 25 ชิ้น
- สำหรับวางลงบน 6 หรือ 12 well plates หรือขนาดอื่นๆ และสามารถวางบนจานเพาะเลี้ยงเซลล์
A 2 well silicone insert with a defined cell-free gap, suitable for wound healing, migration assays, 2D invasion assays, and co-cultivation of cells
- Complete solution for wound healing experiments, requiring only a few steps from sample preparation to image analysis
- Reproducible experiments owing to: a defined 500 µm cell free gap, no leakage during cultivation, and no material being left behind after the insert’s removal
- 25 pieces in a transport dish
- Used for self-insertion into 6 or 12 well plates, or other formats
Not to be used without transferring the Culture-Inserts
Applications
Specifications
| Number of wells | 2 |
| Outer dimensions | 8.4 x 8.4 x 5 mm (w x l x h) |
| Volume per well | 70 µl |
| Growth area per well | 0.22 cm² |
| Coating area per well | 0.82 cm² |
| Width of cell free gap | 500 µm +/- 100 µm |
| Material | Biocompatible silicone |
| Bottom | No bottom – sticky underside |
Technical Features
- Culture-Insert 2 Well in a transport petri dish for self-insertion
- Biocompatible silicone material with an adhesive underside
- Transferable to any flat and clean surface
- Has to be placed in an appropriate cell culture vessel before use, such as the µ-Slide 2 Well, 6 well plates, or 12 well plates
- Clean surface after insert removal without remaining material
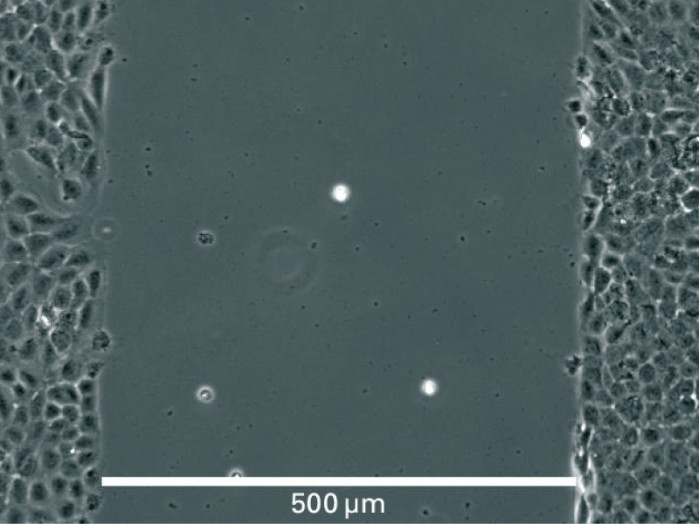
Principle for Wound Healing and Migration Assays
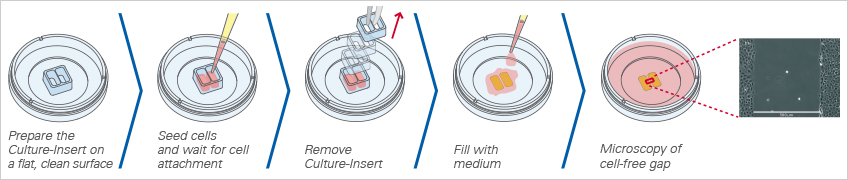
Visualization of Cell Migration in Wound Healing Assays
Wound healing and migration assays are done by seeding cells into the Culture-Insert 2 Well. After cell attachment, a cell-free gap is created in which the cell migration can be visualized.
Cell Invasion of Co-Culture Assays
The Culture-Insert 2 Well can also be used for seeding two different cell types. After the removal of the insert, the cell fronts can be analyzed for their invasional behavior.
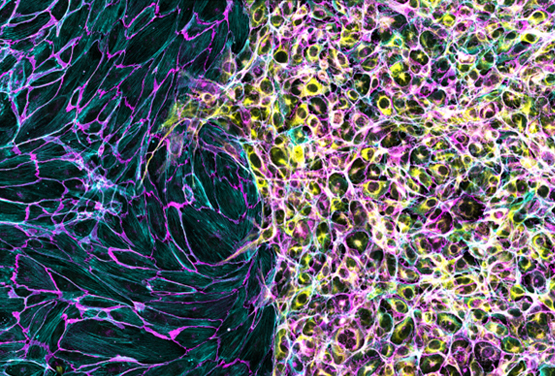
Endothelial cells (left) and trophoblast cells (right) were cultured in an ibidi µ-Dish 35 mm, high with a Culture-Insert 2 Well. Cells were allowed to migrate towards each other after removal of the insert prior to fixation, staining, and imaging. Cells were stained with an antibody against VE-cadherin (magenta), phalloidin for F-actin (cyan), and Cytokeratin 8 (yellow). Image used with permission from Derek Sung.
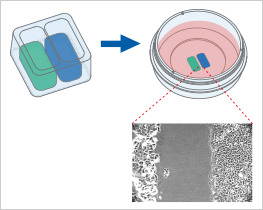
Culture-Inserts vs. Scratch Assay
At first glance, the methods of scratching and placing a Culture-Insert seem to be two very similar approaches to create a cell-free gap. However, at a closer look, these two methods differ in important aspects that could influence the outcome of the assay:
| ibidi Culture-Inserts | Scratch assay | |
| Gap creation | Cell seeding into designated areas | Scratching the cell surface with a needle or pipet tip |
| Gap size | Defined | Varying (i.e., not reproducible) |
| Gap surface residues | No | Extracellular matrix residues possible |
| Vessel surface damage | No | Yes, due to mechanical scratching on the surface |
| Cell damage | No | Yes, due to scratching the cells |
| Segregation & signaling of necrotic/apoptotic cells | Low | High |
| Internal reference * | Yes | No |

* The internal reference addresses the question of whether or not two opposite cell fronts influence each other. By using the Culture-Inserts, it is possible to measure the speed of:
A: a cell front that is opposite another cell front; and
B: a single cell front that does not have an opposite cell front.
The ibidi Culture-Inserts provide improved reproducibility when compared with a scratch assay
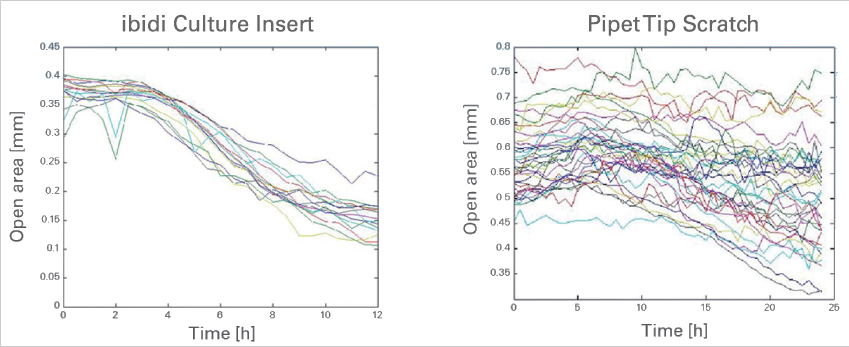
Time-dependent changes of the open area due to cell migration. A comparison between gap creation by using the ibidi Culture-Insert 2 Well (left) and by scratching with a pipet tip. Data provided by M. Börries, University Freiburg, Germany.