ibidi : Collagen Type I, Rat Tail or Bovine
สารละลายคอลลาเจน ชนิด I จาก bovinerat tail คุณภาพสูง สำหรับการเพาะเลี้ยงเซลล์ (เช่น เจล 3 มิติ โครงสร้าง และการเคลือบ)
- ให้โครงสร้างเมทริกซ์นอกเซลล์ (ECM) ในร่างกาย
- คอลลาเจนพื้นเมือง ที่ไม่เปปซิไนซ์สำหรับการสร้างแบบจำลอง ECM ทางชีวภาพในเมทริกซ์เจล
- การเกิดพอลิเมอไรเซชันอย่างรวดเร็วช่วยอำนวยความสะดวกในการกระจายเซลล์ในเจล 3 มิติ
การใช้งาน
- การเพาะเลี้ยงเซลล์ 3 มิติในเมทริกซ์ที่เหมือนในร่างกาย
- การถ่ายภาพเซลล์แบบสดในสภาพแวดล้อม 3 มิติ
- การสร้างโครงนั่งร้านสามมิติสำหรับออร์แกนอยด์และสเฟียรอยด์
- ตรวจ Chemotaxis ในเจลคอลลาเจน 3 มิติโดยใช้ µ-Slide Chemotaxis
- การสอบวิเคราะห์การย้ายถิ่นของเซลล์ของเซลล์แขวนลอย (เช่น เม็ดเลือดขาว)
- การเคลือบหลอดเลือดเพาะเลี้ยงเซลล์ด้วยชั้นคอลลาเจนบางๆ เพื่อส่งเสริมการเกาะติดของเซลล์
- การศึกษาความแตกต่างของเซลล์ในสภาพแวดล้อม 2 มิติกับ 3 มิติ
- เหมาะสำหรับกล้องจุลทรรศน์เรืองแสง นื่องจากออโตฟลูออเรสเซนซ์ต่ำ
คุณสมบัติทางเทคนิค
- โปรตีนบริสุทธิ์คุณภาพสูง จาก rat tendons
- กระบวนการผลิตที่มีมาตรฐานสูง พร้อมการควบคุมคุณภาพที่เข้มงวด
- กระบวนการผลิตที่มีความอ่อนละมุนสูง
- มีโปรโตคอลแบบละเอียด เพื่อให้ง่ายต่อการจัดการ
จัดส่งด้วย Cool Pack และการเก็บรักษาที่อุณหภูมิ –20°C เพื่อคุณภาพและศักยภาพในการทำซ้ำที่ชัดเจน - มีให้เลือก 2 ความเข้มข้น: 5 มก./มล. สำหรับการใช้งานมาตรฐาน และสารละลายเข้มข้นสูง 10 มก./มล. สำหรับการใช้งานพิเศษ
- ระดับการเชื่อมขวางตามธรรมชาติสูงสุดในตลาด (เกรดคุณภาพสูงสุด)
- ความหนืดสูง แนะนำให้ใช้ปิเปตพิเศษสำหรับสารละลายที่มีความหนืดสูง
- ออโตฟลูออเรสเซนซ์ต่ำ
A high-quality collagen type I (bovine or rat tail origin) for cell culture applications (e.g., 3D gels, scaffolds, and coating)
- Provides in vivo-like extracellular matrix (ECM) structures
- Non-pepsinized, native collagen for modelling biological ECM in gel matrices
- Fast gelation facilitates optimal cell distribution in 3D gels
- Concentration / Volume: 5 mg/ml; 1 x 5 ml Trial Pack
Product Variation: Rat Tail
Applications
- 3D cell culture in in vivo-like matrices
- Live cell imaging in a 3D environment
- Creating a 3D scaffold for organoids and spheroids
- Preparation of 3D collagen gels following Application Note 26: “Preparation of Collagen I Gels” (PDF)
- Chemotaxis assays in 3D collagen gels using the µ-Slide Chemotaxis
- Cell migration assays of suspension cells (e.g., leukocytes)
- Coating of cell culture vessels with a thin collagen layer to promote cell attachment
- Cell differentiation studies in a 2D versus 3D environment
- Highly suited for fluorescence microscopy due to low autofluorescence
Technical Features
- High-quality purified protein from rat tendons or bovine hides
- Highly standardized manufacturing process with stringent quality control
- Mild manufacturing process for highest levels of nativity
- Detailed protocol for easy handling in Application Note 26: “Preparation of Collagen I Gels” (PDF)
- Shipping on cool packs for well-defined quality and reproducibility
- Available in 2 concentrations: 5 mg/ml for standard applications and a high-concentrated solution with 10 mg/ml for special applications
- Highest level of natural cross-linking on the market (highest quality grade)
- High viscosity: special pipettes for highly viscous solutions are recommended
- Low autofluorescence
Specifications
| Collagen I, Rat Tail (50201, 50202, 50203, 50204, 50205, 50206) | Collagen I, Bovine (50301, 50302, 50303) | |
| Source material | Rat tail tendon | Bovine dermis |
| Appearance | Optically clear viscous liquid | |
| Extraction | Acid, non-pepsinized | |
| Sterility | Sterile, for cell culture | |
| Quality | Tested negative for bacterial and fungal contamination | |
| Growth factors | None | |
| Formulation | Supplied in 17.5 mM acetic acid | Supplied in 0.1 M acetic acid |
| pH | 3.4–4.2 | 3.3–3.7 |
| Functional control | 3D gelling and 2D coating test in cell culture | |
| Storage | –20°C, avoid multiple freeze and thaw cycles | 2–8°C |
Please note that this product is for research use only. If you are interested in a GMP grade collagen, please contact us at: licensing@ibidi.de

Using Collagen as an in Vivo-Like Matrix for 3D Cell Culture
Collagen is the main component of connective tissue and is abundant in the mammalian body. It is widely used in 3D cell culture for modeling biological extracellular matrix, or as a coating for promoting cell attachment.
Many cells naturally grow in a three-dimensional environment. When cultured in vitro, cells are attached to a flat 2D surface and might behave differently than when they are inside a 3D gel matrix. In many cases, a 3D environment more closely resembles an in vivo situation, which should be considered when planning cell culture assays.
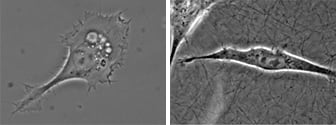
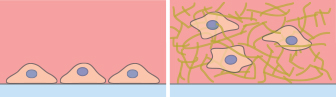
Microscopy and schematic of adherent HT-1080 cancer cells on a 2D surface (left), and embedded into a 3D Collagen Type I, Bovine matrix (right).
The Collagen Structure
Collagen is a fibrous protein that consists of three α-chains. They combine to create a rope-like triple helix, thus providing tensile strength to the extracellular matrix (ECM). The α-chains contain GXY repeats that include glycine (G), which is an amino acid that fits well into the triple helix. Typically, X and Y represent hydroxyproline and proline, which are key components for collagen stability. The triple helices aggregate and form fibrils in a self-organized manner. In vivo, the fibrils gelate into fibers to form tissue such as tendon or dermis.
The collagen that is used for in vitro experiments is acid- or enzyme-extracted from animal tissue. Under acid conditions (low pH), the fibers dissociate into soluble fibrils. In some extraction methods, the enzyme pepsin is used to facilitate the collagen extraction. However, pepsin also digests the collagen to a certain extent, which leads to a significant decrease in native fiber crosslinking activity.
Unlike pepsin-extracted collagen (usually from bovine skin), the ibidi Collagens are acid-extracted. This, and the very mild manufacturing process, preserve a maximal nativity.
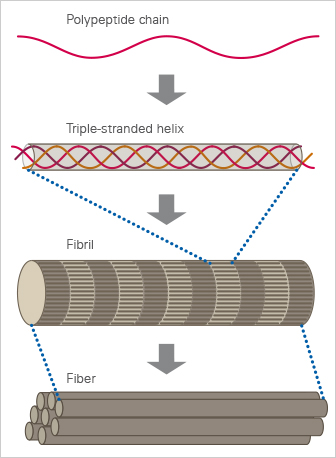
The structure of collagen.
Collagen Gelation
In an acidic solution, the collagen fibrils exist in an unoriented way. Only hydrogen bonds form between the collagen fibrils, which give the solution its high viscosity. Changing to a neutral pH causes the fibrils to gelate into fibers. This creates a three-dimensional collagen gel. The level of nativity of the collagen used defines the level of fiber cross-linking in the collagen network. Native (non-pepsin extracted) collagens exhibit a high level of crosslinking, while pepsinized collagen solutions form a less in vivo-like gel.
For detailed gelation protocols, please read Application Note 26: “Preparation of Collagen I Gels” (PDF).
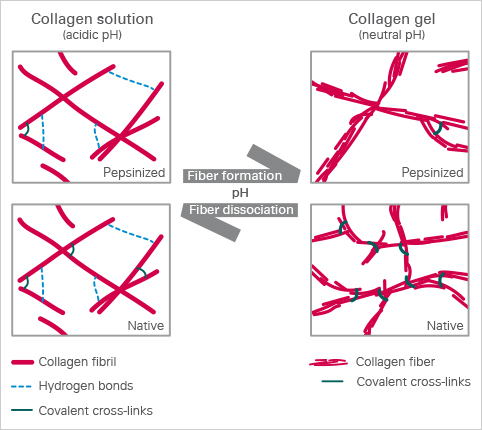
The ibidi collagens have a very high viscosity due to the high protein concentration, especially at 10 mg/ml. The pH-driven gelation changes its characteristic properties as follows:
| Non-Gelated Collagen | Gelated Collagen | |
| Macroscopic optical appearance | Clear to slightly opalescent | White, turbid |
| Microscopic optical appearance | Clear, no structures | Network of fibrils, building fibers that are visible under the microscope |
| Viscosity | Very high, but still flowing | Not flowing; small deformations (e.g., by pipet tips) will rupture the structure |
| Pipetting | Can be pipetted with special pipettes for highly viscous liquids | Defined pipetting is not possible, any mechanical force will destroy the structure and the mechanical properties |
What Are the Differences Between ibidi’s Collagen Type I, Rat Tail
and Collagen Type I, Bovine?
Generally, the Collagen Type I, Rat Tail and the Collagen Type I, Bovine from ibidi are equally suitable for experiments requiring a collagen-based matrix and can be chosen based on personal preference. Since both types are acid-extracted and non-pepsinized, the main difference is the species and the tissue, from which the collagens are extracted. There are small differences in the optical gel appearances. Typically, a rat tail gel exhibits a denser fiber network with less prominent fibers and a smaller pore size. A bovine gel is usually easier to visualize in the phase contrast microscope because the fibers are more bundled, which leads to a larger pore size. Further differences are found in the table of specifications above.

Comparison of hydrogels prepared with Collagen Type I, Rat Tail (left) and Collagen Type I, Bovine (right), each at 2.0 mg/ml in the µ-Slide 15 Well 3D, filled with 10 µl gel per well. Phase contrast imaging on an inverted Nikon microscope with a 20x objective.
Further Reading
Molecular Cell Biology, James Darnell et al. ISBN 0-7167-2380-8
Wolf K, et al. (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201(7):1069–84. 10.1083/jcb.201210152.
Read article
Application Examples
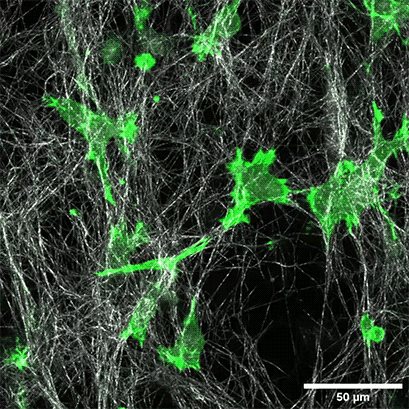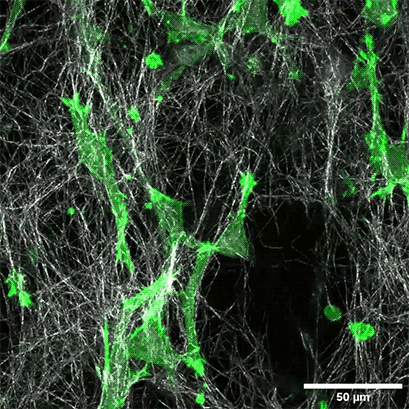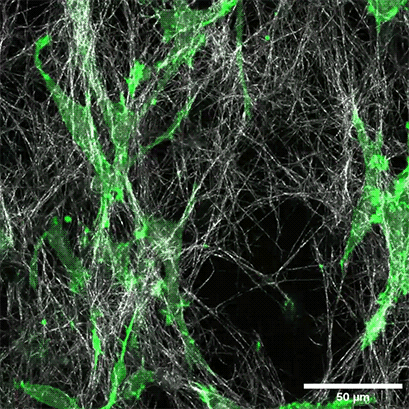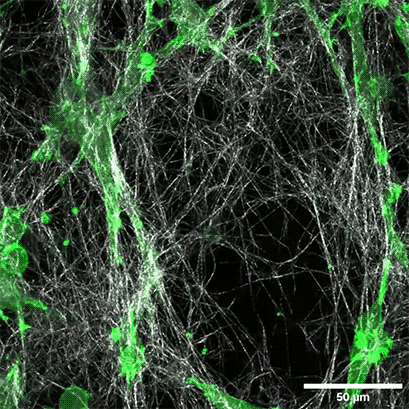
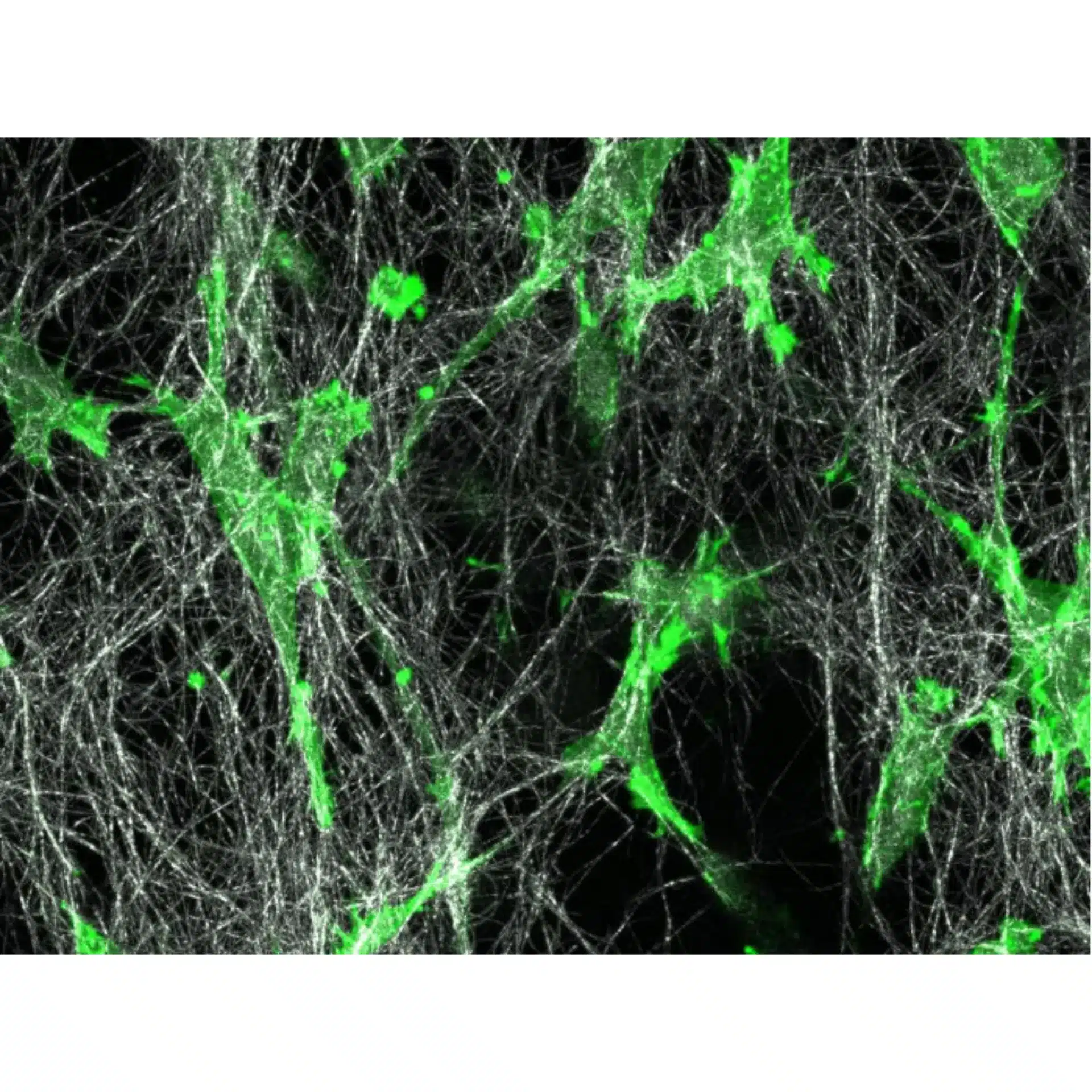
LifeAct-expressing HT-1080 cells (green) were seeded in a 1.5 mg/ml Collagen Type I, Rat Tail, layer (white) in the µ-Slide Chemotaxis. Cell migration was documented by taking a photo every 300 seconds on a Zeiss Confocal Microscope LSM 880 AxioObserver using a water immersion objective lens 40x/1.2.

Live cell imaging of HT-1080 LifeAct-GFP2 cells in a DMEM-based Collagen Type I, Bovine gel (2 mg/ml) on an ibidi Polymer Coverslip. Z-stack with color-coded fluorescence, indicating the distance from the substrate. Imaged with the ZEISS Lattice Lightsheet 7 (LLS7) microscope.

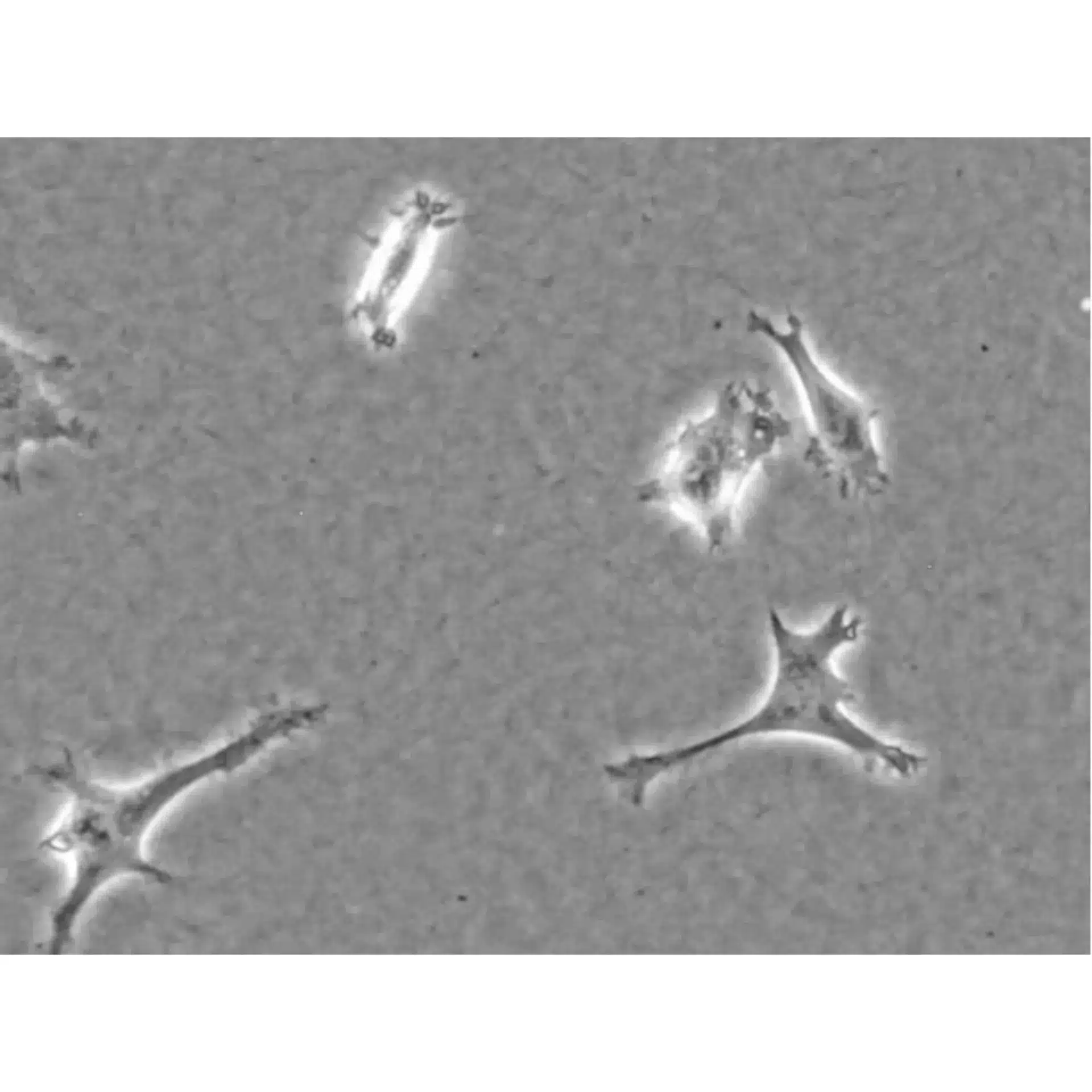
Live cell imaging of HT-1080 cells in a DMEM-based Collagen Type I, Bovine (2 mg/ml) in the µ-Slide Chemotaxis. Phase contrast microscopy in a 5 min interval, using a 20x objective.
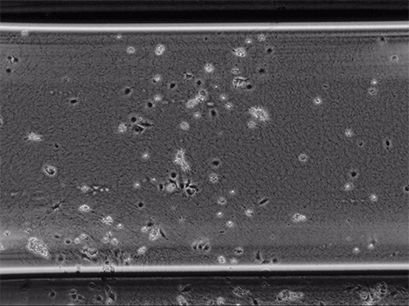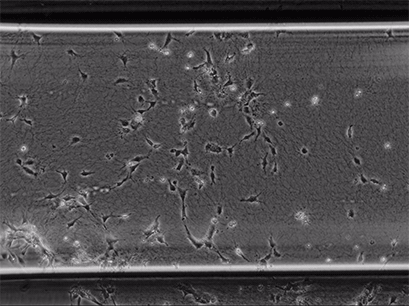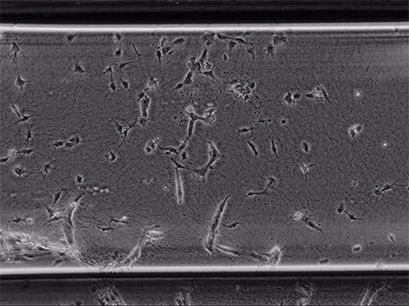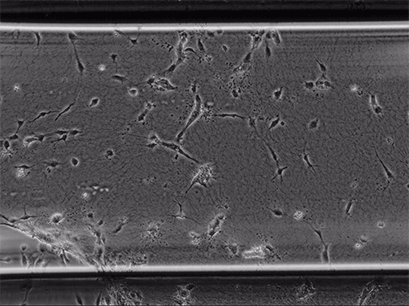
Live cell imaging of Human Umbilical Vein Endothelial Cells (HUVEC) embedded in a 1.5 µg/ml Collagen Type I, Rat Tail, gel in the µ-Slide Chemotaxis, migrating towards fetal calf serum. Note: the cells connecting to each other form strings during the chemotaxis process. Phase contrast, 4x objective lens, 24 hours.
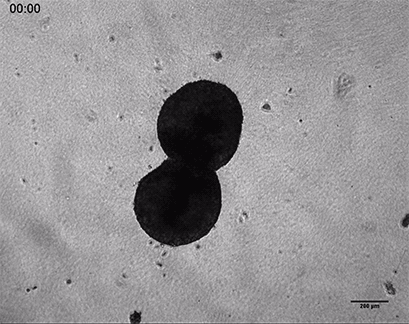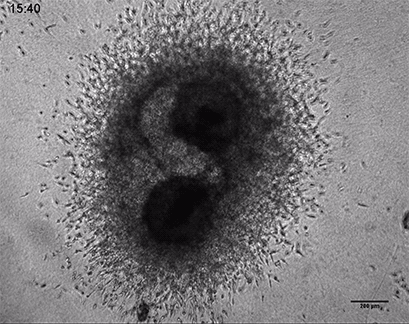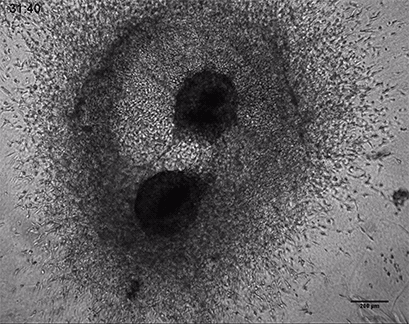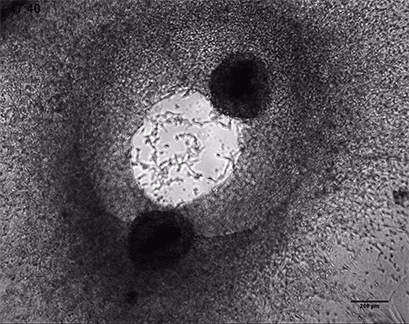
Invasive human fibrosarcoma cancer spheroids (HT-1080) were embedded into Collagen Type I, Rat Tail, gel. The invasion into the gel matrix was recorded for 48 hours in the µ-Slide 8 Well. 4x objective lens, brightfield.
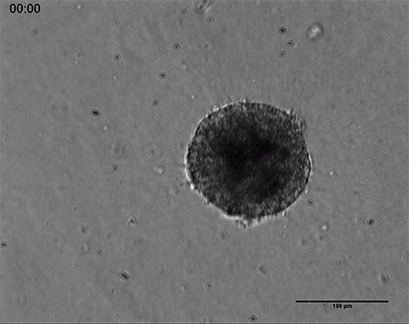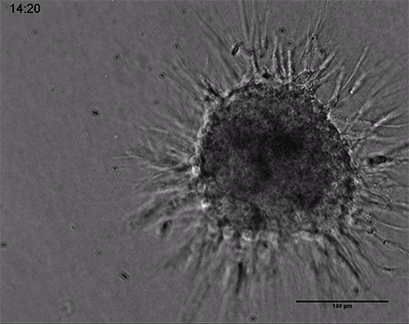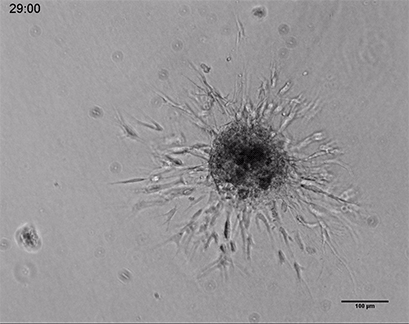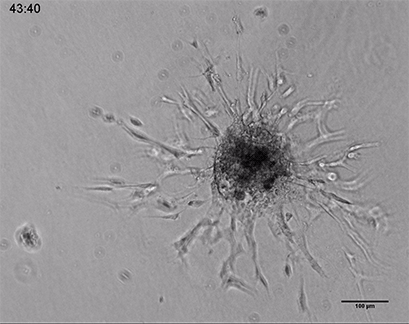
Live cell imaging of a spheroid of Human Umbilical Vein Endothelial Cells (HUVEC), embedded into a 3D gel made of Collagen Type I, Rat Tail. Collagen gel (1 mg/ml) made with full Endothelial Cell Growth Medium (PromoCell) according to Application Note 26 “Fabrication of Collagen I Gels”. VEGF-165 (50 ng/ml) was added to the medium to induce sprouting activity. The sprouting process into the gel matrix was recorded for 44 hours in the µ-Slide 8 Well. 10x and 4x objective lens, brightfield.
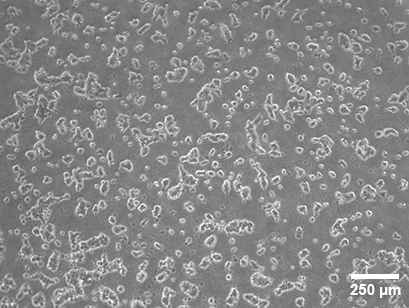
MCF-7 cells on RPMI-based Collagen Type I, Bovine (1 mg/ml) 20 hours after seeding on the gel layer in the µ-Slide 15 Well 3D, filled with 10 µl gel per well. Phase contrast microscopy with a 4x objective.