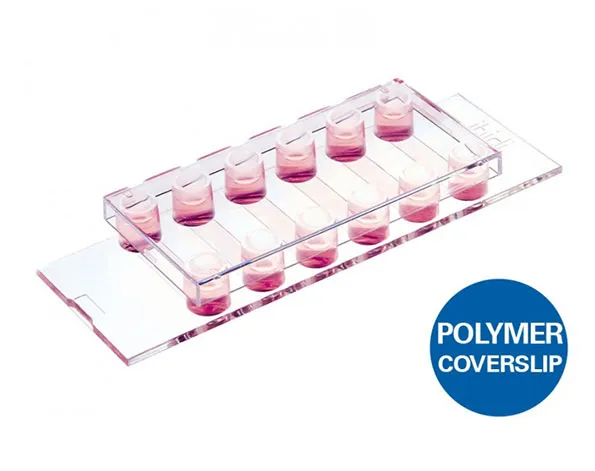
µ-Slide แบบ 6 ช่องสำหรับทำงาน flow assays และสำหรับ immonofluorescence
- สำหรับงาน immunofluorescence
- เซลล์กระจายตัวในช่องว่างอย่างทั่วถึง
- ประหยัดค่าใช้จ่ายในการทำการทดลองเนื่องมาจากใช้เซลล์จำนวนน้อย และใช้ reagent ในปริมาณน้อย
- มี 15 ชิ้น/ 1 กล่อง หรือ 90 ชิ้น/ 1 กล่อง
A 6 channel μ-Slide suitable for flow experiments and for immunofluorescence assays
- All-in-one chamber that simplifies immunofluorescence protocols
- Homogeneous cell distribution over the channel surface, regardless of handling practices
- Cost-effective experiments with small numbers of cells and low volumes of reagents
- Surface Modification: Uncoated: #1.5 polymer coverslip, hydrophobic, sterilized
Pcs./Box: 15 (individually packed) - Surface Modification: ibiTreat: #1.5 polymer coverslip, tissue culture treated, sterilized
Pcs./Box: 90 (individually packed)
Applications
- Immunofluorescence assays and live cell imaging
- Mounting immunofluorescent samples using the ibidi Mounting Medium and the ibidi Mounting Medium With DAPI
- Real-time imaging under either static or flow conditions
- Parallel screenings using multichannel pipettes
Specifications
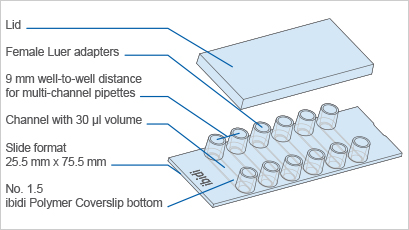
| Outer dimensions (w x l) | 75.5 x 25.5 mm² |
| Adapters | Female Luer |
| Number of channels | 6 |
| Channel volume | 30 µl |
| Channel height | 0.4 mm |
| Channel length | 17 mm |
| Channel width | 3.8 mm |
| Volume per reservoir | 60 µl |
| Height with/without lid | 8.7 mm/7.5 mm |
| Growth area | 0.6 cm² per channel |
| Coating area using 30 µl | 1.2 cm² per channel |
| Bottom: ibidi Polymer Coverslip | |
Technical Features
- 30 µl channel volume, which saves on reagent consumption
- Easy connection to existing tubes and pumps via female Luer adapter
- Fully compatible with high resolution fluorescence microscopy
- Defined shear stress and shear rate level
- Available as a Bulk Pack with 90 individually packed µ-Slides per box
- Available with a non-adhesive Bioinert surface: µ-Slide VI 0.4 Bioinert, specifically suitable for 3D applications, such as spheroids and suspension cells
- Available with a µ-Patterned surface with defined cell adhesion for single-cell and multi-cell applications
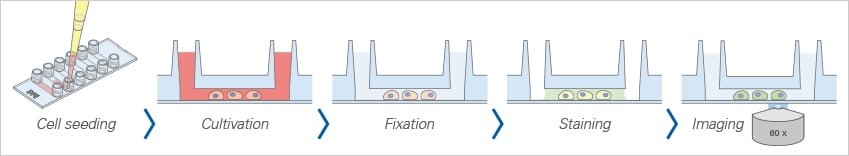
All-in-One-Chamber for Fast Immunofluorescence Protocols
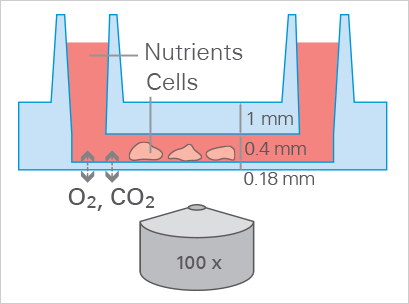
Optimal Cell Growth in the µ-Slide VI 0.4
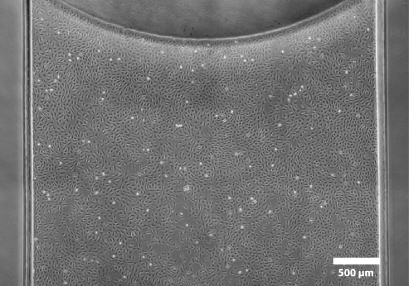
The entire observation field is in excellent phase contrast.
Experimental Examples
Super-Resolution Microscopy (STED) of the Actin Cytoskeleton
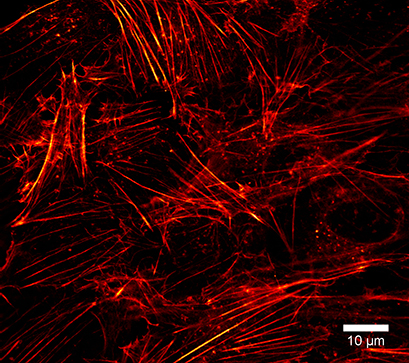
The µ-Slide VI 0.4 is compatible with super-resolution microscopy methods, such as simulated emission depletion (STED) microscopy. Using LifeAct-TagGFP2 Protein, the actin cytoskeleton can be visualized in detail. In this experiment, fixed Rat1 fibroblasts were incubated with LifeAct-TagGFP2 protein in a µ-Slide VI 0.4, ibiTreat. STED microscopy was performed to create a super-resolution image.
Super-resolution microscopy of the actin cytoskeleton in Rat1 fibroblasts using LifeAct-TagGFP2 Protein. Microscopy was performed on the STEDYCON super-resolution STED nanoscopy system (Abberior Instruments GmbH, Göttingen, Germany) with a Plan-Neofluar 100x/1.4 objective lens.
Fluorescence Microscopy for Mitochondria Visualization
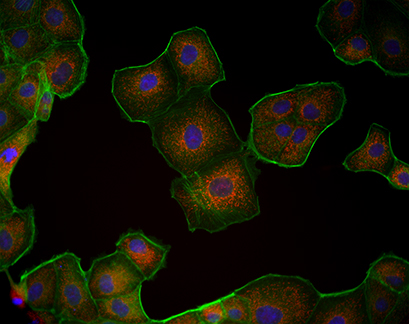
The µ-Slide VI 0.4 is compatible with a variety of fluorescence microscopy methods. In this experiment, Madin-Darby Canine Kidney (MDCK) cells were cultured in a µ-Slide VI 0.4 and their mitochondria were visualized using MitoTracker.
Fluorescence microscopy of MDCK cells cultured in a µ-Slide VI 0.4. Mitochondria (MitoTracker, red), Actin cytoskeleton (Phalloidin, green), nuclei (DAPI, blue).
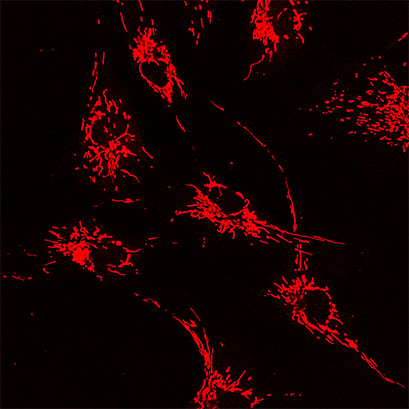
In this experiment, the µ-Slide VI 0.4 was used to acquire confocal images of human BJ fibroblast cells. The cells were transfected with Drp-1 siRNA and stained with MitoTracker Red to visualize fused mitochondria.
Confocal microscopy image of human BJ fibroblast cells cultured in the ibidi μ-Slide VI 0.4. MitoTracker Red (red) was used to visualize mitochondria. Image by Fulya Dal Yontem, Halic University Medical Faculty, Istanbul, Turkey.
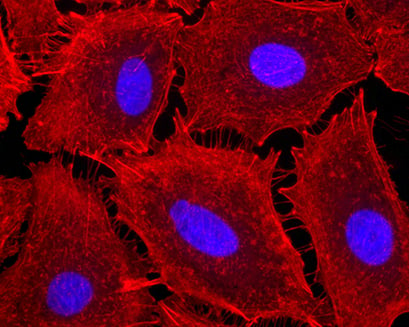
Fluorescence Microscopy for Actin Visualization
In this example, A549 cells (alveolar epithelial cells) were cultured on a μ-Slide VI 0.4, ibiTreat, to study the effect of pro-inflammatory stimuli on cytoskeletal rearrangement. Cells were exposed to endotoxin (LPS) for 4 hours and stained with fluorescent phalloidin to visualize cell contraction with the emergence of intercellular gaps.
A549 cells grown on a μ-Slide VI 0.4, ibiTreat, exposed to endotoxin (LPS) for 4 hours, and stained with fluorescent phalloidin for the detection of cellular actin (red). 40x magnification. Image by Bernard Fisher, Virginia Commonwealth University, Richmond, VA, USA.